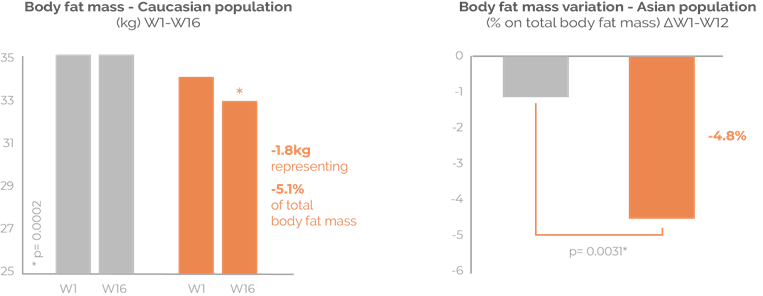
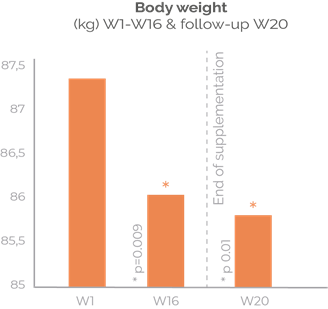
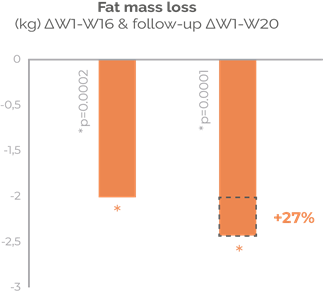
Fat loss for the long-term
Sinetrol® is a natural combination of citrus polyphenols proven to provide the benefits of adipose beiging to increase energy expenditure, and thus amplifying lipolysis and reducing excess fat mass. Sinetrol® improves long-term body composition. Supported with published clinical trials on Asian and Caucasian subjects. 630 mg/day.
Sinetrol® - Key features
-
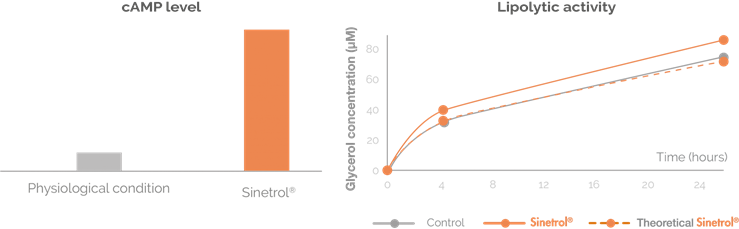
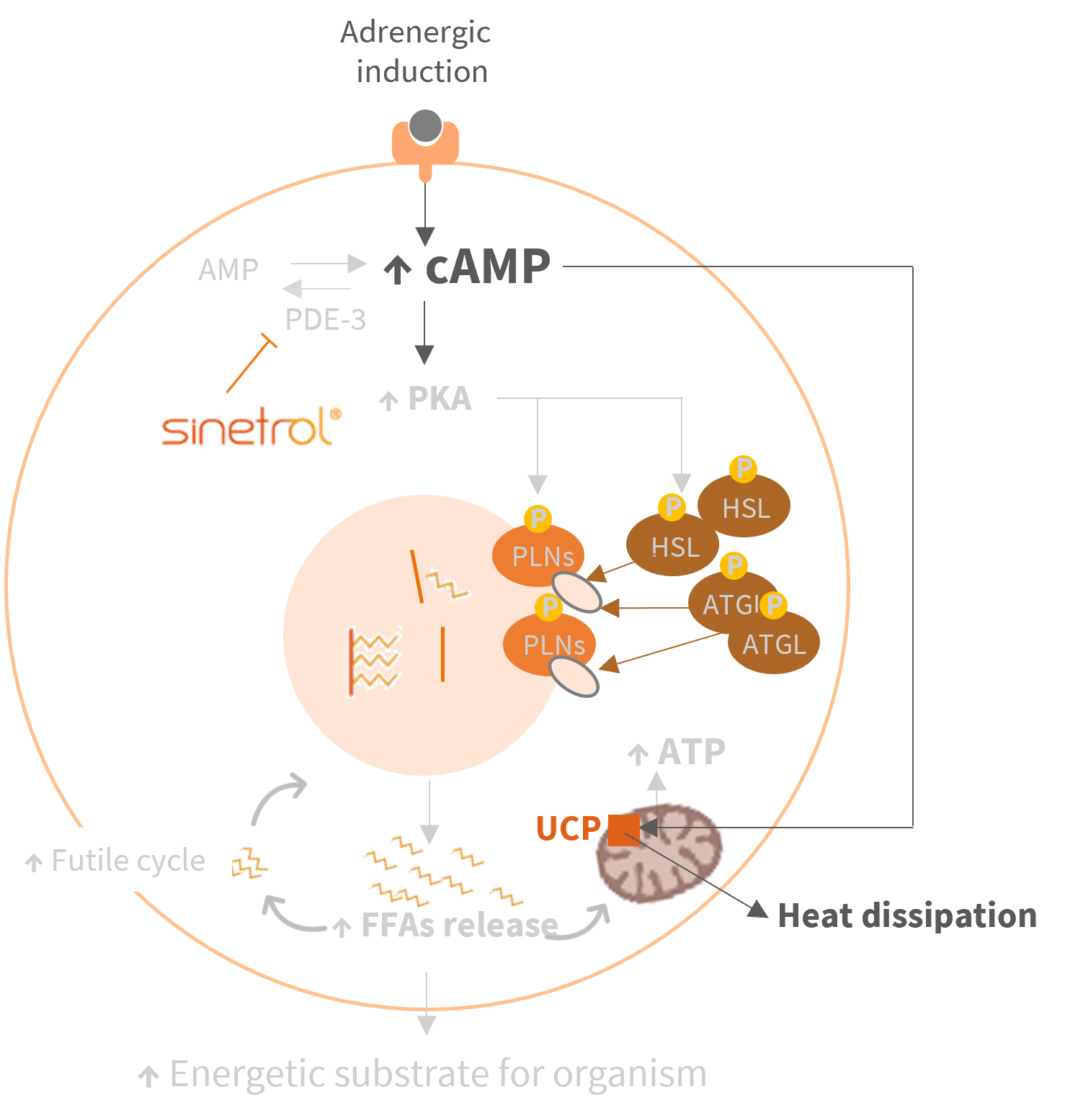
Proven mechanism of action: lipolysis enhancer providing adipose beiging benefits
-
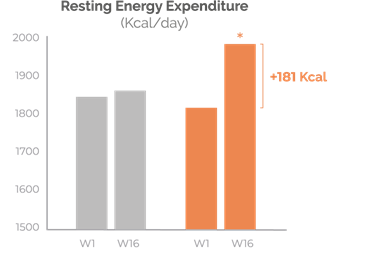
Increase in resting energy expenditure
-
Rebalanced body composition: significant reduction of body fat mass without lean mass loss
-
Significant results on body weight, waist and hip lines
-
Long-lasting benefits: subjects continue to benefit from adipose beiging
Science supporting Sinetrol®
More than 10 years of research have been conducted to support the characterisation, mechanism of action and clinical benefits of Sinetrol® on body composition. Sinetrol® is a botanical ingredient studied in over 300 subjects and its benefits have been demonstrated using gold-standard methodology during interventional studies with follow-up.
Regulatory & Certifications
Non-GMO, Halal, gluten-free, suitable for vegetarians
Approved by MFDS (Korean FDA): may help to reduce body fat
References
CLINICAL TRIALS
Dallas et al. 2008; Phytomedicine
Dallas et al. 2013; Phytother. Res.
Cases et al. 2015; Int. J. Food Sci. Nutr.
Park et al. 2020; J. Med Food
MECHANISTIC STUDIES
Yoo et al. 2016; J. Korean Soc. Food Sci. Nutr
Lee et al. 2017; J. Korean Soc. Food Sci. Nutr.
BIOAVAILABILITY & NUTRIKINETIC
Muralidharan J. et al. 2023; Food Funct.
This website is intended to provide information about Fytexia’s ingredients, used in various food/dietary supplement products around the world. It is only intended for business to business and to provide information to food/dietary supplement professionals and is not designed for the general public. Statements used on this website have not been evaluated by the Food and Drug Administration or any other competent authority. Products are not intended to diagnose, treat, cure or prevent any disease.